| Description |
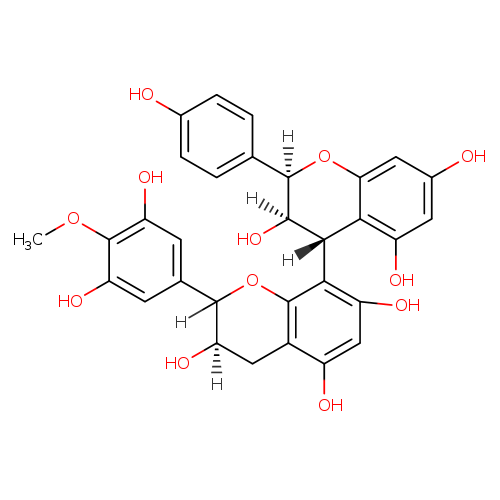
Proanthocyanidins, also known as zangrado or polyhydroxyflavan-3-ol, is a member of the class of compounds known as biflavonoids and polyflavonoids. Biflavonoids and polyflavonoids are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. Proanthocyanidins is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Proanthocyanidins can be found in a number of food items such as roselle, allspice, cocoa bean, and sweet bay, which makes proanthocyanidins a potential biomarker for the consumption of these food products. Proanthocyanidins were discovered in 1947 by Jacques Masquelier, who developed and patented techniques for the extraction of oligomeric proanthocyanidins from pine bark and grape seeds. Often associated with consumer products made from cranberries, grape seeds or red wine, proanthocyanidins were once proposed as factors inhibiting urinary tract infections in women, but this research has been refuted by expert scientific committees . |