| Description |
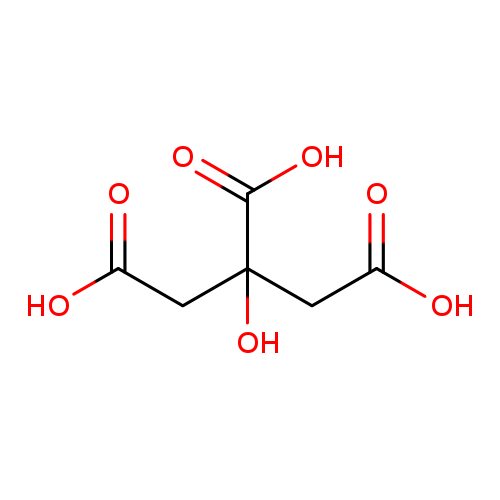
Occurs in the free state in lemons, currants, beetroot etc. and the seeds and juices of many flowers and plants. Commercially produced by large-scale fermentation of sugars using the mould Aspergillus nigus. Constituent of fruit drinks, pharmaceutical syrups. Flavouring ingredient. Primary function as an acid, acidity regulator, antioxidant, preservative and sequestrant. One of the most widely used food additives. Its clean taste makes it applicable in soft drinks, sugar confectionery, preserves, soups and sauces_x000D_
_x000D_
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks. Citric acid is found in many foods, some of which are dill, chanterelle, walnut, and sapodilla. |