tryptophan; l-tryptophan
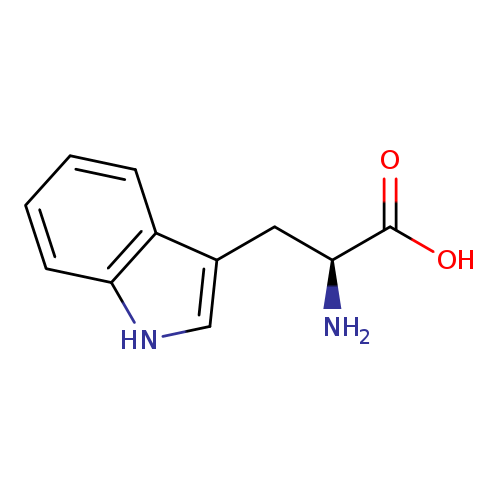
| Name(s) | tryptophan; l-tryptophan |
|---|---|
| Scientific name(s) | 2-amino-3-(3-indolyl)propanoic acid; tryptophan; l-tryptophane; h-trp-oh; (s)-tryptophan; tryptophane; optimax |
| Formula | C11H12N2O2 |
| Molecular mass | 204.229 |
| IUPAC name | 2-Amino-3-(3-indolyl)propanoic acid; (2s)-2-amino-3-(1h-indol-3-yl)propanoic acid |
| INCHI | InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1 |
| SMILE | N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O |
| CAS ID | 73-22-3 |
| PubChem ID | 6305 |
| DrugBank ID | DB00150 |
| CHEBI ID | 16828 |
| Description | Constituent of many plants. Enzymatic hydrolysis production of most plant and animal proteins. Dietary supplement, nutrient_x000D_ _x000D_ Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the D-stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan). |
|---|